Have you ever wondered what exactly Italian salad dressing is from a scientific perspective? Is it a solution, a colloid, or a suspension?
If you’ve ever noticed how the oil and vinegar separate after sitting for a while, you’re already onto something. Understanding this can change how you store, shake, and even enjoy your favorite dressing. You’ll discover the real nature of Italian salad dressing and why it behaves the way it does.
Keep reading to unlock the simple science behind your salad’s perfect flavor blend!
Italian Salad Dressing Components
Italian salad dressing is a blend of several simple ingredients. Each plays a role in the texture and appearance of the dressing. Understanding these components helps explain if the dressing is a solution, colloid, or suspension.
The ingredients mix but do not fully combine into one uniform liquid. This characteristic affects how the dressing behaves over time.
Key Ingredients
The main ingredients include oil, vinegar, and various herbs and spices. Oil and vinegar are immiscible liquids, meaning they do not mix evenly. Herbs and spices add visible particles that float in the mixture. Water is also present in vinegar, helping to create a liquid base.
Other additions like garlic, sugar, and salt dissolve partially, but they do not change the overall mixture type.
Physical Properties
The dressing looks cloudy and uneven. Oil droplets float on top of the vinegar layer before shaking. After shaking, the droplets spread but separate again after standing. Particles like herbs and spices settle slowly over time.
This separation shows the mixture is not uniform. It behaves as a suspension, where solid particles or droplets are dispersed but can settle. Unlike solutions, where particles stay dissolved, Italian dressing separates into layers.
Credit: www.gauthmath.com
Types Of Mixtures
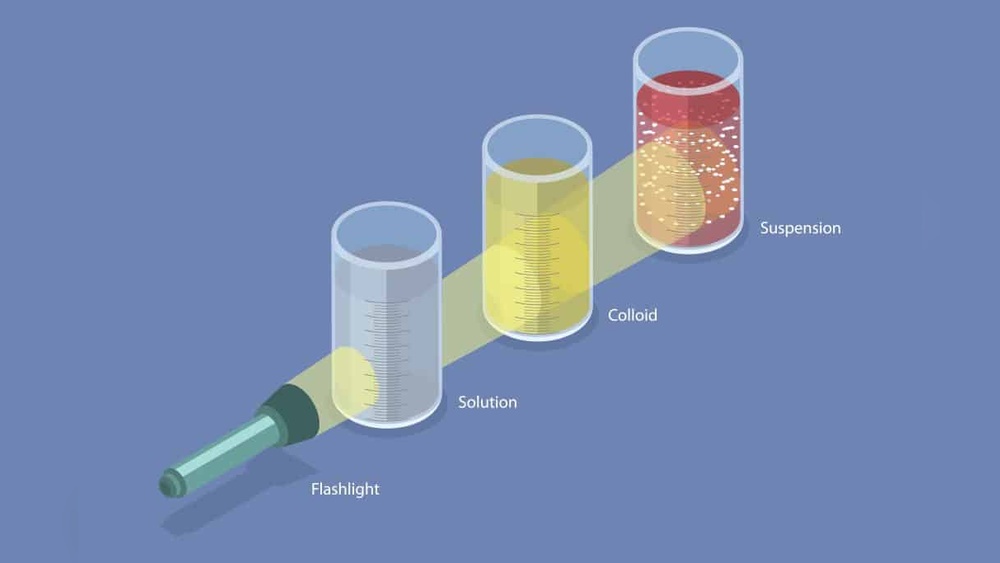
Mixtures are combinations of two or more substances. They can be separated by physical means. Understanding the types of mixtures helps us classify everyday items like Italian salad dressing. Mixtures fall into three main categories: solutions, colloids, and suspensions. Each type has unique properties that affect how the components mix and behave.
Solutions
Solutions are clear and uniform. The particles are very small and dissolve completely. Salt water is a common example. The particles do not settle or scatter light. Solutions look the same throughout and cannot be separated by simple filtering.
Colloids
Colloids have medium-sized particles that stay spread out. These particles do not settle quickly. Milk and fog are examples of colloids. They may appear cloudy or milky. Colloids can scatter light, making them look opaque or translucent.
Suspensions
Suspensions have large particles that are visible to the eye. These particles settle over time. Muddy water and Italian salad dressing are suspensions. The components separate if left standing. Shaking can mix them again, but they will separate later.
Characteristics Of Italian Dressing
Italian dressing is a popular mix of oil, vinegar, herbs, and spices. Its unique texture and appearance come from how these ingredients combine. Understanding its characteristics helps explain if it is a solution, colloid, or suspension.
The mixture looks different depending on how well it is shaken or mixed. Its behavior reveals key details about the particles inside. This guides us to classify the dressing correctly.
Homogeneous Or Heterogeneous?
Italian dressing is a heterogeneous mixture. You can see tiny drops of oil floating in vinegar and spices. The ingredients do not blend into one uniform liquid. This means the mixture has different parts that remain separate.
When left still, the oil rises to the top because it is less dense. The vinegar and spices settle at the bottom. This visible separation proves the dressing is not homogeneous.
Particle Behavior
The particles in Italian dressing are large enough to scatter light and be seen. Oil droplets float throughout the vinegar but do not dissolve. The spices remain suspended but can settle over time.
This particle behavior shows that Italian dressing is a suspension. The particles will separate if the dressing is left undisturbed. Shaking the bottle mixes the particles temporarily, but they do not stay combined forever.
Why Italian Dressing Is A Suspension
Italian salad dressing is a classic example of a suspension. It contains oil, vinegar, and herbs that do not fully mix. Instead, these components remain separate in the mixture. This separation happens because the particles are large enough to be seen and to settle over time. The dressing is not a solution or a colloid due to these visible particles and their behavior.
Particle Size And Settling
The particles in Italian dressing are relatively large. Oil droplets and spice bits float in the vinegar but do not dissolve. Because of this size, gravity pulls them down slowly. Over time, these particles settle at the bottom of the container. This settling is a key sign of a suspension. Solutions have tiny particles that never settle. Colloids have small particles that stay spread out without settling.
Visual Separation Over Time
When left still, Italian dressing clearly separates into layers. The oil rises to the top while vinegar sinks below. The herbs and spices settle at the bottom. This visible separation is easy to notice. Shaking the bottle mixes the particles again, but they will separate soon. This behavior shows the dressing is not uniform. A solution would stay mixed and clear. A colloid would look cloudy but not separate.
Comparing With Colloids And Solutions
Italian salad dressing is a mixture of oil, vinegar, and spices. Understanding its nature requires comparing it with colloids and solutions. These three types of mixtures differ in particle size and behavior. Identifying where Italian salad dressing fits helps explain why it separates over time.
Difference From Solutions
Solutions are uniform mixtures. Their particles are very small and dissolve completely. For example, sugar in water forms a solution. Italian salad dressing is not a solution because oil and vinegar do not mix evenly. The particles in the dressing are visible and separate over time. This makes it a heterogeneous mixture, unlike solutions that stay uniform.
Difference From Colloids
Colloids have particles larger than solutions but smaller than suspensions. These particles stay mixed and do not settle out quickly. Milk is a common colloid example. Italian salad dressing differs because its oil droplets eventually separate and float on top. The particles in the dressing do not remain suspended like in colloids. This shows it is closer to a suspension rather than a colloid.
Credit: www.numerade.com
Practical Implications
Understanding the practical implications of Italian salad dressing’s nature helps in daily use. Since it is a suspension, the ingredients separate over time. This separation affects storage, mixing, taste, and texture. Proper handling ensures the best flavor and consistency for your salads.
Storage And Mixing Tips
Store Italian dressing in a cool, dark place to keep it fresh. Avoid heat and sunlight which speed up separation. Always shake the bottle well before use. Shaking mixes the oil, vinegar, and spices evenly. Use a jar with a tight lid to prevent leaks. Refrigerate after opening to slow particle settling. Stirring gently before serving keeps the dressing uniform.
Effect On Taste And Texture
Separation changes the taste and texture of the dressing. The vinegar may taste stronger if oil settles at the top. Uneven mixing can make some bites sour and others oily. Proper shaking blends flavors for a balanced taste. The texture feels smooth when ingredients are well mixed. A poorly mixed dressing feels greasy or watery on salads.
Other Salad Dressings And Their Classifications
Salad dressings come in many varieties, each with unique mixtures. Understanding their classifications helps explain their behavior and appearance. Some dressings are clear and uniform, while others separate or look cloudy. These differences arise from whether they are solutions, colloids, or suspensions.
Each type of mixture has distinct features. Solutions are completely uniform, colloids have tiny particles that stay mixed, and suspensions contain larger particles that settle over time. Let’s explore examples of these types in common salad dressings.
Examples Of Solutions
Vinaigrette dressings often act like solutions if well mixed and stable. For example, a simple mix of vinegar and water forms a solution. The liquid looks clear, and particles do not separate. Soy sauce is another example of a solution used in some salad dressings. It is uniform and does not separate.
Examples Of Colloids
Creamy dressings such as ranch or blue cheese are colloids. They contain tiny droplets of oil or solid particles evenly spread in water. These particles are small enough to stay mixed without settling. The dressing looks thick and smooth but is not fully transparent. Mayonnaise is a classic colloid dressing.
Examples Of Suspensions
Italian salad dressing is a good example of a suspension. It has visible oil droplets and spices that separate over time. After shaking, it looks mixed but soon separates into layers. Another example is a chunky vinaigrette with herbs and seeds. The particles are large enough to settle if left standing.

Credit: www.teacherspayteachers.com
Frequently Asked Questions
Is Italian Salad Dressing A Solution, Suspension, Or Colloid?
Italian salad dressing is a suspension. It contains visible particles that separate and settle over time, making it a heterogeneous mixture.
What Kind Of Solution Is Italian Dressing?
Italian dressing is a heterogeneous mixture classified as a suspension. Its oil, vinegar, and spices separate over time, requiring shaking before use.
Is Salad Dressing A Solution Or Suspension?
Salad dressing is a suspension because it contains visible particles that separate and settle over time. It is a heterogeneous mixture, not a uniform solution.
What Is Italian Dressing Classified As?
Italian dressing is classified as a suspension. It is a heterogeneous mixture with particles that can settle over time. The oil, vinegar, and spices separate if left undisturbed, distinguishing it from solutions or colloids.
Conclusion
Italian salad dressing is a classic example of a suspension. Its oil, vinegar, and spices separate when left still. The mixture is not uniform like a solution. Tiny particles do not stay mixed as in colloids. Shaking helps blend the ingredients temporarily.
Over time, the particles settle at the bottom. This shows the dressing is a suspension, not a colloid or solution. Understanding this helps explain why you must shake it before use. Simple science behind a tasty salad dressing.